- Amal Augustine
- February 7, 2026
Ultimate Redox Potential Order MCQS Class 12 – Best Electrochemistry Practice
Electrochemistry in Class 12 Chemistry places strong emphasis on understanding oxidation–reduction processes, and among these concepts, redox potential order holds a central position. Aspirants preparing for board exams and competitive tests often practice redox potential order mcqs class 12 to strengthen their ability to compare different redox systems accurately.
Redox potential order mcqs class 12 refers to the arrangement of species based on their standard reduction potentials. A higher reduction potential indicates a greater tendency to gain electrons, meaning the species acts as a stronger oxidising agent. Conversely, species with lower or more negative reduction potentials tend to lose electrons easily and therefore act as stronger reducing agents. This idea is repeatedly tested through redox potential order mcqs class 12.
Understanding redox potential order helps aspirants predict the feasibility of redox reactions. When one species has a higher reduction potential than another, it can oxidise the latter. This predictive power is essential not only for numerical problems but also for theoretical reasoning questions often framed like redox potential order mcqs class 12.
A strong conceptual link exists between redox potential order and electrochemical cells. In any galvanic cell, the electrode with the higher reduction potential functions as the cathode, while the one with the lower reduction potential becomes the anode. Questions based on this relationship frequently appear in redox potential order mcqs class 12, making clarity on this point crucial.
Redox potential order also plays a key role in identifying oxidising and reducing agents. For example, among halogens, fluorine has the highest reduction potential, making it the strongest oxidising agent. Such trends are often tested directly or indirectly in redox potential order mcqs class 12, requiring students to remember periodic trends alongside electrochemical data.
Another important application of redox potential order is in displacement reactions. A metal with a lower reduction potential can displace another metal from its salt solution if the displaced metal has a higher reduction potential. Mastery of this idea ensures confidence when solving redox potential order mcqs class 12 that involve reaction feasibility.
In aqueous solutions, redox potential order also determines which species will be preferentially discharged during electrolysis. Whether hydrogen is evolved or a metal is deposited depends on comparative reduction potentials. This practical relevance makes redox potential order mcqs class 12 especially important from an applied chemistry perspective.
Redox potential order further helps in understanding corrosion and its prevention. Metals with more negative reduction potentials corrode more easily, which explains why sacrificial protection works. These real-life applications reinforce the concepts tested through redox potential order mcqs class 12.
Fuel cells and batteries also rely heavily on redox potential order. The voltage generated by a cell is directly related to the difference in reduction potentials of the two electrodes. Questions built around such concepts are common in redox potential order mcqs class 12, especially those linking theory with numerical interpretation.
Another subtle but important aspect is the difference between standard conditions and non-standard conditions. While redox potential order is usually discussed using standard values, real systems may deviate due to concentration or pressure changes. Even so, a firm understanding of standard ordering remains the foundation for solving redox potential order mcqs class 12.
Aspirants often struggle when multiple species are involved in a single comparison. Systematic practice helps them quickly identify the strongest oxidising and reducing agents without confusion. This skill is sharpened primarily through repeated exposure to redox potential order mcqs class 12.
From an examination strategy point of view, redox potential order questions are scoring because they test logic more than lengthy calculations. Once concepts are clear, such questions can be answered quickly and accurately, giving an edge in exams dominated by redox potential order mcqs class 12.
Redox Potential Order MCQs Class 12 with Solved Answers
An electrochemical cell has two half-cell reactions:
A²⁺ + 2e⁻ → A, E° = 0.34 V
X → X²⁺ + 2e⁻, E° = –2.37 V
The cell voltage will be:
a) 2.71 V
b) 0.03 V
c) –2.71 V
d) –2.03 V
Answer: a
2.
Which of the following statement is not correct about an inert electrode in a cell?
a) It does not participate in the cell reaction
b) It provides surface either for oxidation or reduction
c) It provides surface for conduction of electrons
d) It provides surface for redox reaction
Answer: d
3.
The emf of a galvanic cell constituted with electrodes
Zn²⁺ | Zn (–0.76 V) and Fe²⁺ | Fe (–0.41 V) is:
a) –0.35 V
b) –1.17 V
c) 0.35 V
d) 1.17 V
Answer: c
4.
Which one of the following hydrogen electrodes has potential more than zero?
a) Pt | ½H₂ (1 atm) | HCl (2 M)
b) Pt | ½H₂ (1 atm) | HCl (0.1 M)
c) Pt | ½H₂ (1 atm) | HCl (0.5 M)
d) Pt | ½H₂ (1 atm) | HCl (1 M)
Answer: a
5.
Given E°(M⁺/M) = 0.52 V and E°(N⁺/N) = 0.25 V, for the cell
M | M⁺ || N⁺ | N :
a) Standard EMF = –0.27 V
b) Standard EMF = 0.27 V
c) Standard EMF = –0.77 V
d) Overall reaction is spontaneous
Answer: a
6.
For the disproportionation reaction
2Cu⁺ → Cu²⁺ + Cu,
E° is (given E° Cu²⁺/Cu = 0.34 V and Cu²⁺/Cu⁺ = 0.15 V):
a) 0.49 V
b) –0.19 V
c) 0.38 V
d) –0.38 V
Answer: c
7.
The strongest oxidizing and reducing agents respectively are:
a) F₂ and I⁻
b) Br₂ and I⁻
c) Cl₂ and Br⁻
d) Cl₂ and I₂
Answer: a
8.
In the cell
Zn + 2H⁺ → Zn²⁺ + H₂,
addition of H₂SO₄ to the cathode compartment will:
a) Lower E and shift equilibrium left
b) Lower E and shift equilibrium right
c) Increase E and shift equilibrium right
d) Increase E and shift equilibrium left
Answer: c
9.
The emf of a Daniell cell containing 0.1 M ZnSO₄ and 0.01 M CuSO₄ is:
a) 1.10 V
b) 1.16 V
c) 1.13 V
d) 1.07 V
Answer: d
10.
To increase the emf of the cell
Zn + Cl₂ → Zn²⁺ + 2Cl⁻ :
a) Increase [Zn²⁺]
b) Decrease [Zn²⁺]
c) Increase [Cl⁻]
d) Decrease Cl₂ pressure
Answer: b
11.
The reduction potential of an electrode can be increased by:
a) Increasing electrode area
b) Decreasing temperature
c) Increasing temperature
d) Decreasing metal ion concentration
Answer: b
12.
E° for Cr³⁺ + 3e⁻ → Cr is:
a) –0.741 V
b) –1.324 V
c) –0.476 V
d) 0.741 V
Answer: a
13.
Sequence of metal deposition at cathode during electrolysis is:
a) Ag, Mg, Cu
b) Mg, Cu, Ag
c) Ag, Hg, Cu
d) Cu, Hg, Ag
Answer: c
14.
Equilibrium constant for
Cu + 2Ag⁺ → Cu²⁺ + 2Ag (E° = 0.46 V) is:
a) 4.0 × 10¹⁵
b) 2.0 × 10¹⁰
c) 2.0 × 10¹⁰
d) 4.0 × 10¹⁰
Answer: a
15.
Hg cathode forms sodium amalgam instead of H₂ because:
a) Hg is more inert
b) More voltage required to reduce H⁺ at Hg
c) Na dissolves in Hg
d) Higher H⁺ concentration
Answer: b
16.
EMF of reaction
Fe²⁺ + Zn → Zn²⁺ + Fe is:
a) 1.28 V
b) –1.28 V
c) 0.35 V
d) –0.35 V
Answer: d
17.
EMF of cell Cu²⁺/Cu and Zn²⁺/Zn is:
a) 1.10 V
b) –1.10 V
c) –0.42 V
d) 0.42 V
Answer: d
18.
Which statement is correct?
a) X₂ stronger oxidising agent
b) Y₂ stronger oxidising agent
c) X⁻ stronger reducing agent
d) Both equal
Answer: a
19.
Standard emf of cell
Cr₂O₇²⁻ + Fe²⁺ → is:
a) 1.33 + 0.77
b) 1.33 – 0.77
c) –(1.33 + 0.77)
d) –1.33 + 0.77
Answer: b
20.
For a cell with n = 2 and E° = 0.59 V, K equals:
a) 10¹⁰
b) 10⁵
c) None
d) 10²⁰
Answer: d
21.
Redox potential for Mn³⁺ + 3e⁻ → Mn is:
a) 0.33 V
b) 1.69 V
c) –0.28 V
d) –0.85 V
Answer: c
22.
Standard emf of
Fe²⁺ + Sn → Fe + Sn²⁺ is:
a) 0.30 V
b) 0.58 V
c) –0.38 V
d) –0.30 V
Answer: d
23.
E° for Cu²⁺/Cu equals:
a) 0.30 V
b) 0.325 V
c) 0.650 V
d) 0.150 V
Answer: b
24.
Favourable redox reaction is:
a) I₂ reduced
b) No reaction
c) Fe²⁺ oxidised
d) I⁻ oxidised
Answer: d
25.
Reducing power order is:
a) Y > Z > X
b) Y > X > Z
c) Z > X > Y
d) X > Z > Y
Answer: c
26.
KNO₃ is used in salt bridge because:
a) K⁺ faster
b) NO₃⁻ faster
c) Both move equally
d) High solubility
Answer: c
27.
Strongest reducing agent is:
a) Cu(s)
b) K⁺(aq)
c) Zn²⁺(aq)
d) Fe(s)
Answer: b
28.
Correct cell reaction is:
a) 2Br⁻ + Cl₂ → 2Cl⁻ + Br₂
b) Br₂ + 2Cl⁻ → 2Br⁻ + Cl₂
c) Br₂ + Cl₂ → products
d) 2Br⁻ + 2Cl⁻ → products
Answer: a
29.
Standard oxidation potential of silver is:
a) –0.80 V
b) 0.80 V
c) 0.40 V
d) –0.40 V
Answer: a
30.
Most characteristic oxidation states are:
a) Pb +4, Sn +2
b) Pb +2, Sn +2
c) Pb +4, Sn +4
d) Pb +2, Sn +4
Answer: d
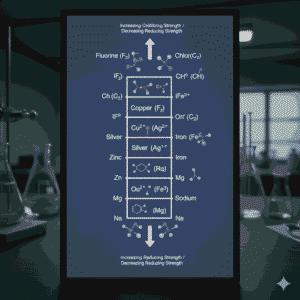
Conclusion on Redox Potential Order MCQs Class 12
In conclusion, redox potential order is a foundational concept that connects multiple chapters of electrochemistry. Whether it is predicting reaction direction, identifying agents, analysing cells, or understanding real-life applications, this concept remains indispensable. Consistent conceptual clarity and revision ensure success in tackling redox potential order mcqs class 12, making them an effective tool for mastering Class 12 electrochemistry.

Amal Augustine is the founder of ExQuizMe, a dynamic learning and quiz platform built to make education engaging, competitive, and fun. A passionate learner and an academic achiever, Amal completed his schooling at Government HSS Manjapra, graduating with 92.5% in Computer Science. He later earned his degree from St. Stephen’s College, University of Delhi, one of India’s most prestigious arts and science institutions.
Currently, Amal is pursuing his Master’s degree at National Sun Yat-sen University, Taiwan, where he continues to deepen his interest in research and technology. Throughout his school and college years, he won 50+ national-level interschool and collegiate quiz competitions, was
Beyond academics, Amal Augustine is an avid reader of science journals, a dedicated research student, and a technology enthusiast who loves programming and exploring the world of Computer Science. Through ExQuizMe, he aims to make learning accessible, enjoyable, and empowering for students across the globe.
